How Many O Atoms Are Present in Sodium Chlorate
How many sulfur atoms are present in 256 g of Al2S2O33. Chlorine is A MOLECULAR ELEMENT the smallest unit of a covalently bonded element that is capable of independant existence.

How To Find The Number Of Atoms In Naclo4 Sodium Perchlorate Youtube
29 For the reaction C 2H2 CH4 how many grams of hydrogen are required to produce 177 moles of methane CH4.
. So here we go. Use the factor label method. A sample of oxygen gas 02 weighs 300 grams.
How many atoms are in sodium chlorate. Calculate the mass of sodium chlorate that must be decomposed to form 65 g of oxygen according to the following equation. CO2 is a chemical compound composed two oxygen atoms covalently bonded to a single carbon atom Sodium is NOT a MOLECULAR ELEMENT rather it is also an atomic element an element composed of single unbounded atoms.
We assume you are converting between grams Sodium Chlorate and mole. How many atoms are present in 358 grams of sodium. On the exam you have to write the equivalent statements and show the correct setup to get credit.
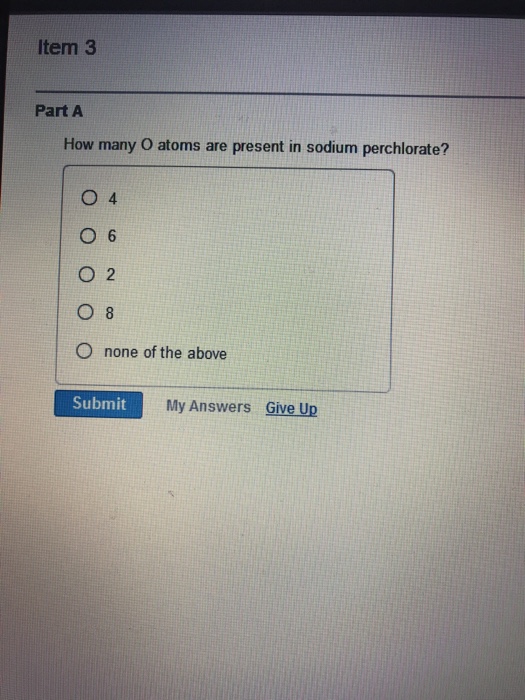
How many oxygen atoms are present in sodium chlorate a 3 b 6 c 4 d 1 e None of the above. So Mass of the Oxygen is here 32 As one oxygen atoms atomic mass is 16. 937 x 10 atoms 358 g Na 1 mol Na 2299 g Na 60210 23 atoms Na 937 x 10 23 atoms 1 mole Na.
There are 2 oxygen atoms in 1 formula unit of sodium chlorite NaClO2. The small number after the element symbol is c. How many grams Sodium Chlorate in 1 mol.
How many moles of NaCl are in 71 grams of this mixture. Molecular weight of Sodium Chlorate or mol The molecular formula for Sodium Chlorate is NaClO3. How many moles are present in 517 grams of sodium chlorate NaCIO.
Chlorine Reactions Key takeaways Chlorate I is a negative ion with the formula. Ammonium hydrogen carbonate NH4CO3 c. 2 NaClO3s 2 NaCls 3.
2 A sample of oxygen gas O2 weighs 300 grams. Does clo3 react with water. You can view more details on each measurement unit.
Sodium chlorite NaClO2 b. 7 What is the. A 2 B 6 C 4 D 1 E none of the above.
How many grams of helium are in a balloon with 840 moles of helium atoms. There are one chlorine atom and three oxygen atoms in the chlorate ion. A 20-g sample of washing soda Na2CO3 10H2O has carbon atoms.
The SI base unit for amount of substance is the mole. Sodium chlorate decomposes into sodium chloride and oxygen gas as seen in the equation below. There is one atom of Na one atom of Cl and three atoms of O for.
How many molecules of and how many atoms of O are present in this sample. 3 A mixture of san and salt is found to be 42 NaCl by weight. The formula for magnesium chloride is MgCl2.
2NaClO3 -- 2NaCl 3O2 How many moles of NaClO3 were needed to produce 44 moles of O2. 2NaClO3 2 Nacl 3O2 So we are getting 3 moles of O2 from from 2 moles of NaClO3 therefore we will get 18 moles of O2 from 12 moles of NaClO3. Arrenhasyd and 5 more users found this answer helpful.
02 18 molecules of O 18 ye atoms of O 78 18 8. NaClO3 is sodium chlorate. How many O atoms are present in sodium chlorite.
1 Chemical Foundations 2 Atoms Molecules And Ions 3 Stoichiometry 4 Types Of Chemical Reactions And Solution Stoichiometry 5 Gases 6 Thermochemistry 7 Atomic Structure And Periodicity 8 Bonding. It is also known as hypochlorite. To get the answer first we need to understand how sodium chlorate decomposes by such we can able to calculate.
What is the formula for magnesium chlorate. 120 grams1 mole4003 grams60221023 atoms1 mole1811024 atoms. 44 The charge of a vanadium ion in the compound V2O5 is.
To find the total number of atoms in NaClO4 Sodium perchlorate well add up the number of each type of atom. And the chlorate ion are represented respectively as. 13 How many oxygen atoms are present in sodium chlorate a 3 b 6 c 4 d 1 e None from CHEM 22 at Pasadena City College.
A MgClO3 B Mg2ClO3 C MgClO32 D Mg2ClO33 E none of the above. Sodium chlorate NaClO3 or ClNaO3 CID 516902 - structure chemical names physical and chemical properties classification patents literature biological. How many molecules of O2 and how many atoms of O are present in this sample.
To determine the number of atoms in a 120 g sample of helium we must divide by the molar mass and multiply by Avogadros number. The answer is 10644097. 216 x 10 21 atoms D.
H4 and H3 are hydrogens just add then and youve counted the number of Hydrogens. How many oxygen atoms are present in 20g of washing soda. 937 x 10 atoms.
How many O atoms are present in sodium chlorite. Round your answer to the nearest whole number. 1 How many moles are present in 517 grams of sodium chlorate NaClO3.
Of Atoms. Also there is a -1 charge on the ClO 3 ion.
What Is The Number Of Moles Of Nitrogen Atoms Present In 16 Grams Of Ammonium Nitrate Quora
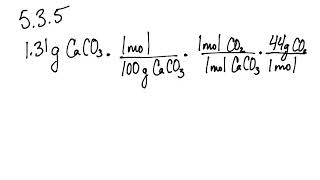
5 3 Calculating Reaction Yields Problems Chemistry Libretexts

The Crystal Lattice Structure Of Potassium Chloride Kcl A Salt Which Is Formed Due To The Attraction Of K Crystal Lattice Structure Chemistry Potassium Atom
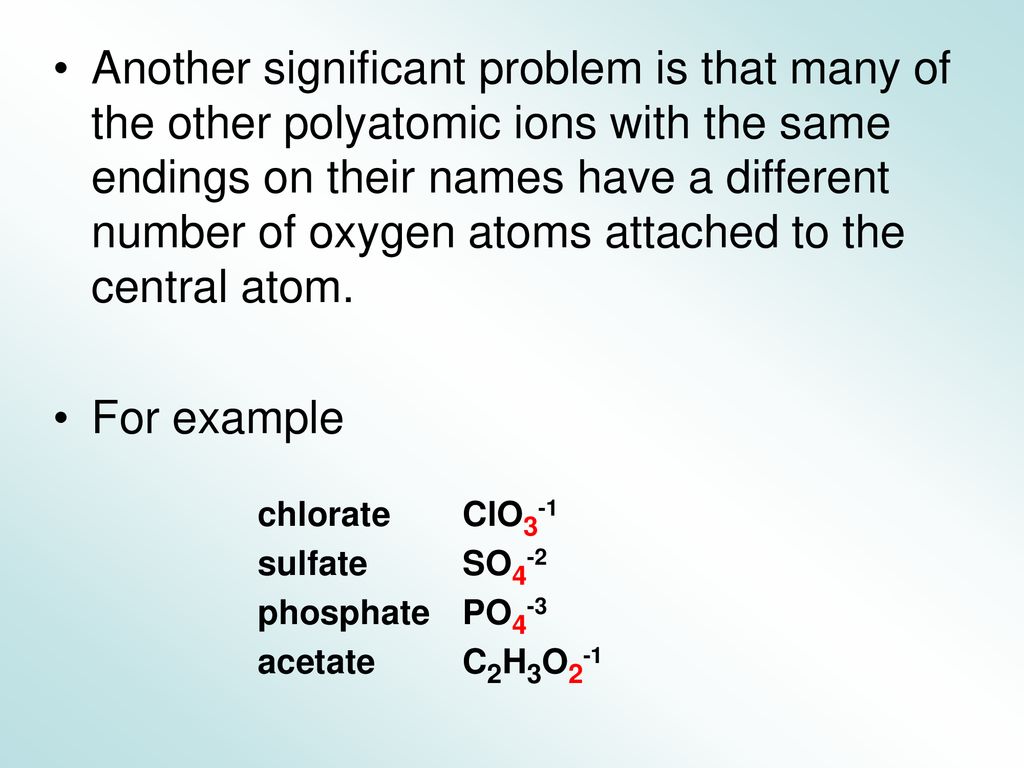
Aqueous Acids And Their Salts Ppt Download

5 3 Calculating Reaction Yields Problems Chemistry Libretexts
What Is The Number Of Oxygen Atoms In One Mole Of Cuso4 5h2o Quora
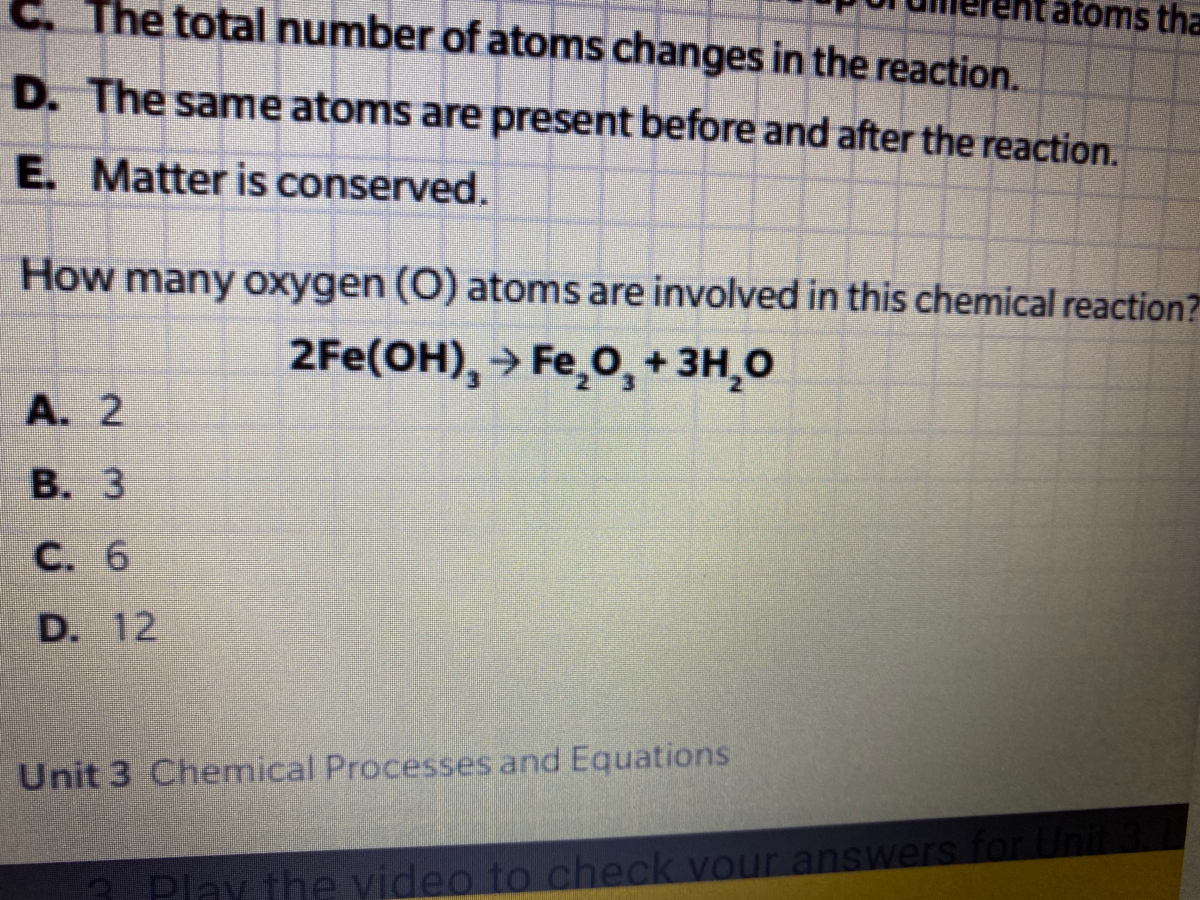
Answered How Many Oxygen O Atoms Are Involved Bartleby

Solved Item 3 Part A How Many O Atoms Are Present In Sodium Chegg Com
How Many Atoms Of Oxygen Are Present In 50g Of Caco3 Quora

Solved A Write Out The Formula For Ammonium Chlorate And Chegg Com

1 Binary Compounds Only Two Different Elements Present Examples Nacl Mgcl 2 Al 2 O 3 Ionic Covalent H 2 O Co So 2 Cbr 4 Ionic Metal Ion Positive Or Ppt Download

Calculate The Formal Charge On Each Of The Oxygen O Atoms Labeled A B And C In The Following Brainly Com
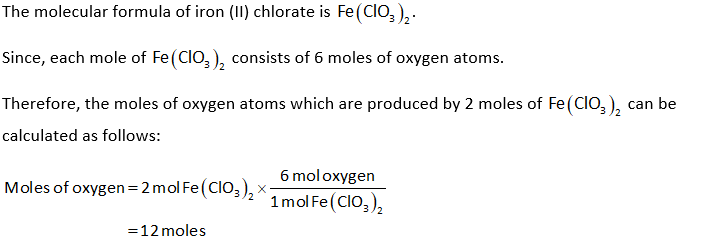
Answered Determine The Number Of Moles Of Oxygen Bartleby

How To Find The Number Of Atoms In N2o5 Dinitrogen Pentoxide Youtube

Solved Calculate Each Of The Following Quantities A Mass G Of 8 35 Mathrm Mol Of Copper I Carbonate B Mass G Of 4 04 Times 10 20 Molecules Of Dinitrogen Pentoxide C Amount Mol And Number Of

Oxygen Discovery Symbol Properties Uses Facts Britannica

13 How Many Oxygen Atoms Are Present In Sodium Chlorate A 3 B 6 C 4 D 1 E None Course Hero

Comments
Post a Comment